Uncovering Bacterial Survival Strategy
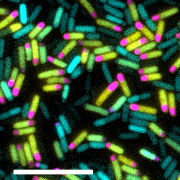
The starving bacterial cells that have begun spore formation divide asymmetrically into large (yellow) and small (pink) chambers. The small chamber corresponds to the future spore. The cells that fail to sporulate (blue) die eventually. Scale bar: 10μmIf you feel hungry, you want to eat something. If there is nothing to eat, what can you do? In the face of catastrophic events, bacteria, like people, make plans for survival.
Scientists at the University of Houston and Rice University have uncovered an elaborate and sophisticated strategy that allows bacterial cells to begin preparing survival plans in advance of an anticipated catastrophic event. The results published December 11 in the Proceedings of the National Academy of Sciences.
The paper examines findings related to Bacillus subtilis, a common soil bacterium that is harmless to humans. “When B. subtilis cells are hungry, they form spores, which can survive for decades in the environment,” said Masaya Fujita, associate professor of biology and biochemistry at UH and principal investigator on the National Science Foundation-funded project.
During spore formation, or sporulation, the cell undergoes changes in shape; asymmetric division begins and gives rise to two distinct cell types, a large mother cell and a small forespore. Each cell type contains the same genome but expresses a different subset of genes.
As sporulation progresses, the smaller cell is engulfed and nurtured by the larger cell, which eventually dissolves to release the mature spore into the environment. The gene expression program for sporulation involves approximately 500 genes out of the 4,200 genes in B. subtilis.
“For decades, the functions of many of the individual genes have been characterized in detailed molecular terms, but how these work together as a system to orchestrate the sporulation decision remains a mystery,” Fujita said. “We wanted to find a way to determine the cell’s decision-making process for sporulation.”
However, the UH and Rice scientists faced a problem; traditional methods used to investigate the interactions between individual genes do not work well with the wild-type strain of B. subtilis.
To overcome these limitations, Fujita and UH graduate student Seram Devi have created a new synthetic genetic system that decouples the unknown sporulation signal from the downstream events in the sporulation gene networks. This new system, called artificial sporulation initiation (ASI), allows well-controlled sporulation gene regulation by adding a drug called IPTG, resulting in the artificially-induced sporulation.

From left, Masaya Fujita and UH graduate student Seram Devi examine B. subtilis cells grown on an agar plate. The cells are later analyzed using fluorescence microscopy. The team used ASI in combination with a computational model created by Rice University’s Oleg Igoshin, an assistant professor of bioengineering, and Rice graduate student Jatin Narula. The findings revealed that the decision-making process is considerably more complex than expected.
One long-standing hypothesis suggests that the master regulator Spo0A is responsible for kicking off the process of spore formation, so it has been considered as the key player of cell fate decision.
“However, the cells expressing Spo0A only kick off the sporulation process and begin making preparations, but the cells are not yet committed to this survival strategy,” Fujita said.
The team found that the ultimate decision process is a result of a series of nested “feed forward” loops – network motifs in which one master regulator controls another by directly regulating its amount and indirectly regulating its activity.
Using such integrated and sophisticated genetic networks, the cells can process information, and if needed, change their mind, though without a brain. This strategy allows them to make an accurate decision under unpredictable environmental conditions. Thus, the cells can postpone their final decision-making until the point-of-no-return.
The scientists hope that insights gained into the cellular mechanisms used by B. subtilis will not only close a gap in the understanding of the fundamentals of bacterial physiology and regulation, but will also provide a basis for the design of new antibacterial agents.
“This same strategy may be applicable to other spore-forming bacteria that are harmful to humans, such as Clostridium sp., B. cereus, and B. anthracis. These bacteria are involved in tetanus, food spoilage, and anthrax,” Fujita said.
- Prepared by Masaya Fujita and Kathy Major, College of Natural Sciences and Mathematics, with excerpts from Jade Boyd’s Rice University news release.